Megaloptera
Alderflies, dobsonflies, fishflies
Atilano Contreras-Ramos

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The order Megaloptera, formerly considered a suborder (Sialodea) of Neuroptera, is generally considered to be among the most primitive of the holometabolous insect orders. It contains two families, the Sialidae (alderflies) and the Corydalidae, the latter subdivided into the Corydalinae (dobsonflies) and the Chauliodinae (fishflies). The fauna of Megaloptera consists of about 300 extant species worldwide (New and Theischinger 1993). A list of all megalopteran genera is available here.


A typical dobsonfly male with elongated mandibles. Corydalus imperiosus Contreras-Ramos (Corydalidae: Corydalinae), Misiones, Argentina. Photograph copyright © 1997, Atilano Contreras-Ramos
Like Ephemeroptera, Odonata, Plecoptera, and Trichoptera, the order Megaloptera is entirely aquatic, i.e., all--or nearly all--megalopteran species have at least one aquatic stage. Adults of Corydalidae are particularly noteworthy for their frequently large size, and, in many species of the genera Corydalus and Acanthacorydalis, for the extremely elongated mandibles of adult males. Adult fishflies and dobsonflies are generally nocturnal and secretive, while alderflies are diurnal yet not too frequently collected. At the right place and time of the year (e.g., near the edge of clean lakes in Minnesota, U.S.A., around June), however, it is possible to find individuals of the fishfly Chauliodes rastricornis Rambur congregating under lights, or aggregates of alderflies on vegetation during day time.
Characteristics
Adults of Megaloptera can be identified by the enlarged and fan-folded anal area of their hind wings (Borror et al. 1989). Those of Corydalidae are large (forewing longer than 15 mm, wingspan up to 180 mm), pale yellowish to brownish or spotted black, with ocelli, and their 4th tarsal segment is simple; while those of Sialidae are small (forewing 15 mm or less), dark brown to gray and black (sometimes with orange spots on the head), lack ocelli, and their 4th tarsal segment is bilobed.
Larvae are elongate, moderately flattened, prognathous, have a distinct labrum, and measure 10-90 mm when mature. Mouth parts are of the chewing type, well developed. Larvae bear lateral abdominal filaments (on segments 1-8 in Corydalidae, and 1-7 in Sialidae) and either a pair of anal prolegs (Corydalidae) or a single caudal filament (Sialidae). Members of the subfamily Corydalinae also possess tufts of accesory tracheal gills under the lateral filaments of segments 1-7 (Evans and Neunzig 1996, Theischinger 1991).
Life History
Oviposition
Megaloptera adults lay their egg masses on rocks, tree trunks, leaves, and other substrates adjacent to water, and the young larvae fall or crawl into the water shortly after hatching. Sialid egg masses are single layered, whereas corydalids lay their eggs in egg masses of one to five layers (Evans & Neunzig 1996).
There does not seem to be a clear preference for oviposition substrates in Neotropical dobsonflies. Nevertheless, a certain selectivity for substrates may be hard to determine in some cases, as egg masses of Chloronia, Corydalus, and Platyneuromus are quite similar (whitish, chalky, coin size). Canterbury (1978) reports a general selection of vegetation hanging over the water for the oviposition of eastern North American sialids, however, some species preferred leaves, and other species oviposited on twigs or branches.
Larval Habitats
Typically, alderfly larvae are associated with lentic habitats (sediments of lakes and depositional zones of streams), while corydalid larvae (hellgrammites) mostly occur in lotic environments (riffles and other erosional zones, from small mountain streams to large rivers). Some Australian fishflies are known from swamps (Theischinger 1991), whereas North American Chauliodes are typically associated with lakes, ponds, and swamps (Cuyler 1958). Canterbury (op. cit.) found that some Sialis species seemed to be confined to one type of aquatic habitat (e.g., small clear streams, larger streams, small woodland ponds, or bays and inlets of lakes). Hayashi (1989), as well, points out a possible habitat seggregation between Parachauliodes japonicus (MacLachlan) and Protohermes grandis (Thunberg) larvae in Japanese streams. The former apparently restrict themselves to shallow edge waters, where they can utilize abdominal respiratory tubes in the event of an oxygen decrease, while the latter prefer the central part of riffles (larvae having gill tufts, but lacking respiratory tubes). On the same token, Geijskes (1984) found in Suriname an apparent preference of Corydalus nubilus Erichson and C. affinis Burmeister to large open rivers, while C. batesii MacLachlan and Chloronia hyeroglyphica (Rambur) seemed to be restricted to small shaded creeks.
Recent findings indicate the occurrence of Chauliodes and Sialis larvae in "unusual" habitats, such as tree holes and purple pitcher plants (Fashing 1994, Hamilton et al. 1996, Pittman et al. 1996). This is additional evidence of this group's larval endurance to cope with potentially inhospitable conditions. This is the case in other fishfly species, such as Neohermes californicus (Walker), whose first instar larvae bury into the substrate of dry intermittent streams during summer in California, remaining in cells until rains reappear in late fall and streams flow once again (Smith 1970).
Larval Development
Alderflies are very difficult to find in tropical climates (Henry et al. 1992). Although they occur through most of the world (with the exception of much of Africa), life histories of species of temperate climates are better known. In Europe, the life cycle of Sialis lutaria L. has been found to take from one to two years and even three years in high altitude lakes (Dall 1989), whereas in North America Sialis species have shown life cycles of one to two years (Azam & Anderson 1969, Pritchard & Leischner 1973). There are 10 larval instars in the development of Sialis under natural conditions (New & Theischinger 1993, Evans & Neunzig 1996).
Evans (1972) estimated that development of western North American fishflies might take from two (Neohermes) to four and five years (Dysmicohermes, Orohermes, Protochauliodes), while Hayashi (1989) estimated a two-year larval period for Parachauliodes japonicus. He (1988a) also determined a two to three year larval period for Protohermes grandis (Thunberg).
Bowles (1990) cites studies on Nearctic Corydalus cornutus (L.) that report life histories of one (southern latitudes) to five years (northern latitudes), the larvae having 10-12 instars. Hayashi (1994) found larval periods that varied from one to three years in Japanese Protohermes, period length depending strongly on water temperature.




Immature Megaloptera. a. larva of Platyneuromus soror (Hagen) (Corydalidae, Corydalinae), Nuevo León, Mexico; b. male pupa of Corydalus cornutus (L.) (Corydalidae, Corydalinae), Alabama, USA; c. female pupa of Nigronia fasciatus (Walker) (Corydalidae, Chauliodinae), Alabama, USA. Photographs copyright © 1997, Atilano Contreras-Ramos
Larval Feeding Ecology
Megalopteran larvae are generalist predators and, at least in captivity, scavengers. Stewart et al. (1973) found larvae of black flies (Diptera: Simuliidae) and net spinning caddisflies (Trichoptera: Hydropsychidae) as the main items in the diet of the dobson fly Corydalus cornutus (L.) hellgrammites in a Texan river, but 17 other aquatic insect groups (in addition to individuals of its own species!) were also eaten. Hayashi (1988b) found larvae of the Japanese fish fly Protohermes grandis to feed on a wide variety of benthic macroinvertebrates including mayflies, stoneflies, and chironomids, showing some degree of cannibalism as well. Similar patterns have been observed for alder flies, although a certain specificity has been detected. For instance, the North American Sialis itascae Ross was observed to prey mostly on ostracods, even when several other prey were available (Lilly et al. 1978, cited by New & Theischinger 1993).
Pupae and Adults
When mature, the larvae leave the water and build a chamber under a rock or log, not too far from the aquatic habitat. In these chambers they spend several days as prepupae (generally about a week), then they molt and become decticous and exarate pupae which are capable of limited movement, including a strong defensive bite. After several more days (8-24 days in Corydalidae, 5-8 days to about a month in Sialidae; New & Theischinger 1993) the adults emerge and the life cycle is completed.
Adults are short-lived and generally do not feed, though they may drink water or sweet solutions. Parfin (1952) recorded an average of eight days for the adult longevity of Nearctic Corydalus cornutus. Mexican adult Platyneuromus soror (Hagen) lived for up to a week in captivity, however actual life span might be longer in nature since specimens had been collected with black light; moreover damage on wings and antennae points out injuries due to confinement conditions (Contreras-Ramos 1999a).
Reproductive Biology
Mating and courtship behavior are better known in Sialidae than in Corydalidae. Several studies on Sialis (New & Theischinger 1993) reported reciprocal signalling, which implies a vertical vibration of the abdomen by males and females. Vibrations allow mutual recognition of species and sex. In some dobsonflies (e.g., Corydalus texanus Banks and Platyneuromus soror), males may use female attractant scents from eversible glands near the genitalia (between 8th and 9th abdominal segments and under the 9th sternum; Evans 1972; Contreras-Ramos 1998, 1999a).
Corydalus males fight when encountering each other (Evans 1972, Parfin 1952). Platyneuromus males only display a threatening position (mandibles open) but do not fight (Contreras-Ramos 1990). Premating behavior in Corydalus involves touching of antennae between male and female while facing one another, as well as male wing fluttering (Parfin 1952, Evans 1972). In Platyneuromus soror, males actively pursue females, at the same time fluttering their wings and lifting their genitalia (10th tergites) above the level of the wings in an "arrogant" position (Contreras-Ramos 1999a).
Fertilization involves the transfer of a gelatinous spermatophore (Hayashi 1992, 1993). This reproductive strategy might cause low levels of sexual selection ("female choice" pattern, sensu Eberhard 1985), resulting in a rather conservative morphology of male genitalia (Contreras-Ramos 1998).
Discussion of Phylogenetic Relationships
The monophyly of Megaloptera is generally accepted, however adult synapomorphies remain obscure (New and Theischinger 1993, Kristensen 1991). Boudreaux (1979) suggested the following traits as apomorphies for the order: (i) wing pterostigma secondarily unpigmented, (ii) loss of the terminal bifurcations of the main veins, (iii) larvae aquatic (the embryonic limb buds become leglike structures into which respiratory tracheae appear to penetrate), (iv) larval maxillary stipes elongated, as in the adult condition. Similarly, the monophyly of each family (and so the sister group relationship of Chauliodinae and Corydalinae) has not been demonstrated explicitly through a phylogenetic analysis. It is, however, a general assumption in current classifications.
Boudreaux (1979) proposed the following features as derived. Corydalidae: (i) enlarged jugal area with a prominent jugal bar in the hind wing, (ii) hind wing folding among the posterior anal veins (not at the jugal fold as in other endopterygotes and Sialidae). Sialidae: (i) adult gula, cervical region, and the prothoracic sternum secondarily desclerotized, (ii) ocelli lost, (iii) 4th tarsomeres strongly bilobed, (iv) claspers of the male genitalia reduced, and (v) larval pygopods (anal prolegs) lost.
Since Sialidae appears to be the group with more specializations, such as the secondary desclerotization of the gular region and the reduction of structures in the male genitalia, among others, there should be caution as to grouping Chauliodinae and Corydalinae on the basis of primitive characters (however this question deserves a thorough phylogenetic analysis of its own).
References
Azam, K. M., and N. H. Anderson. 1969. Life history and habits of Sialis rotunda and Sialis californica in western Oregon. Ann. Ent. Soc. Am. 62: 549-558.
Barnard, K. H. 1931. The Cape alderflies (Neuroptera, Megaloptera). Transactions of the Royal Society of South Africa 19: 169-184.
Barnard, K. H. 1940. Additional records, and descriptions of new species, of South African alder-flies (Megaloptera), may-flies (Ephemeroptera), caddis-flies (Trichoptera), stone-flies (Perlaria), and dragon-flies (Odonata). Ann. Sth. Afr. Mus. 32: 609-615.
Berland, L., and P.-P. Grassé. 1951. Super-ordre des Néuroptéroides, pp. 3-69 In P.-P. Grassé (ed.). Traité de Zoologie, Vol. 10. Mason et Cie., Paris. 975 pp.
Borror, D. J., C. A. Triplehorn, and N. F. Johnson. 1989. An introduction to the study of insects. Saunders College Publishing, Philadelphia. 875 pp.
Boudreaux, H. B. 1979. Arthropod phylogeny. John Wiley & Sons, New York. 320 pp.
Bowles, D. E. 1990. Life history and variability of secondary production estimates for Corydalus cornutus (Megaloptera: Corydalidae) in an Ozark stream. J. Agric. Entomol. 7: 61-70.
Brigham, W. U. 1982. Megaloptera, pp. 7.1-7.12 In A. R. Brigham, W. U. Brigham, and A. Gnilka (eds.). Aquatic insects and oligochaetes of North and South Carolina. Midwest Aquatic Enterprises, Mahomet, Illinois. [837 pp.]
Canterbury, L. E. 1978. Studies of the genus Sialis (Sialidae: Megaloptera) in eastern North America. Unpublished Ph. D. dissertation. University of Louisville, Kentucky. 93 pp.
Chandler, H. P. 1956. Megaloptera, pp. 229-233 In R. L. Usinger (ed.). Aquatic insects of California. University of California Press, Berkeley. 508 pp.
Contreras-Ramos, A. 1998. Systematics of the dobsonfly genus Corydalus Latreille (Megaloptera: Corydalidae). Thomas Say Monographs, Entomological Society of America. Lanham, MD. 360 pp.
Contreras-Ramos, A. 1999a. Mating behavior of Platyneuromus (Megaloptera: Corydalidae), with life history notes on dobsonflies from Mexico and Costa Rica. Ent. News 110: 125-135.
Contreras-Ramos, A. 1999b. List of species of Neotropical Megaloptera (Neuropterida). Proc. Entomol. Soc. Wash. 101: 274-284.
Contreras-Ramos, A. and S. C. Harris. 1998. The immature stages of Platyneuromus (Corydalidae), with a key to the genera of larval Megaloptera of Mexico. J. N. Am. Benthol. Soc. 17(4): 489-517.
Cuyler, R. D. 1958. The larvae of Chauliodes Latreille (Megaloptera: Corydalidae). Ann. Ent. Soc. Am. 51: 582-586.
Eberhard, W. G. 1985. Sexual selection and animal genitalia. Harvard University Press, Cambridge. 244 pp.
Evans, E. D. 1972. A study of the Megaloptera of the Pacific coastal region of the United States. Unpublished Ph.D. dissertation. Oregon State University, Corvallis. 210 pp.
Evans, E. D., and H. H. Neunzig. 1996. Megaloptera and aquatic Neuroptera, pp. 298-308 In R. W. Merritt and K. W. Cummins (eds.). Aquatic insects of North America. Kendall/Hunt Publishing Company, Dubuque, Iowa. 862 pp.
Fashing, N. J. 1994. A novel habitat for larvae of the fishfly Chauliodes pectinicornis (Megaloptera: Corydalidae). Banisteria 3: 25-26.
Flint, O. S., Jr. 1973. The Megaloptera of Chile (Neuroptera). Rev. Chil. Ent. 7: 31-45.
Geijskes, D. C. 1984. Notes on the Megaloptera from the Guyanas, S. Am., pp. 79-84 In J. Gepp, H. Aspöck, and H. Hölzel (eds.). Progress in World's Neuropterology, Grazz. 265 pp.
Gurney, A. B., and S. Parfin. 1959. Neuroptera, pp. 973-980 In W. T. Edmondson (ed.). Fresh-water biology. John Wiley & Sons, New York. 1248 pp.
Hamilton, R., M. Whitaker, T. C. Farmer, A. A. Benn, and R. M. Duffield. 1996. A report of Chauliodes (Megaloptera: Corydalidae) in the purple pitcher plant, Sarracenia purpurea L. (Sarraceniaceae). Journal of the Kansas Entomological Society 69: 257-259.
Hayashi, F. 1988a. Life history variation in a dobsonfly, Protohermes grandis (Megaloptera: Corydalidae): effects of prey availability and temperature. Freshwater Biology 19: 205-216.
Hayashi, F. 1988b. Prey selection by the dobsonfly larva, Protohermes grandis (Megaloptera: Corydalidae). Freshwater Biology 20: 19-29.
Hayashi, F. 1989. Microhabitat selection by the fishfly larva, Parachauliodes japonicus, in relation to its mode of respiration. Freshwater Biology 21: 489-496.
Hayashi, F. 1992. Large spermatophore production and consumption in dobsonflies Protohermes (Megaloptera, Corydalidae). Jpn. J. Ent. 60: 59-66.
Hayashi, F. 1993. Male mating costs in two insect species (Protohermes, Megaloptera) that produce large spermatophores. Anim. Behav. 45: 343-349.
Hayashi, F. 1994. Life-history patterns in 15 populations of Protohermes (Megaloptera: Corydalidae): Effects of prey size and temperature, pp. 227-243 In H. V. Danks (ed.). Insect life-cycle polymorphism. Kluwer Academic Publishers, The Netherlands.
Hayashi, F. 1995. Sialidae (Megaloptera) of Japan. Aquatic Insects 17: 1-15.
Hayashi, F. 1996. Life cycle of Protohermes immaculatus (Megaloptera: Corydalidae) accelerated by warm water overflowing dam. Aquatic Insects 18: 101-110.
Henry, C. H., N. D. Penny, and P. A. Adams. 1992. The neuropteroid orders of Central America, pp. 432-458 In D. Quintero and A. Aiello (eds.). Insects of Panama and Mesoamerica. Oxford University Press, Oxford. 692 pp.
Kristensen, N. P. 1991. Phylogeny of extant hexapods, pp. 125-140 In C.S.I.R.O. (ed.). The insects of Australia, Vol. I. Cornell University Press, Ithaca. 542 pp.
Larsson, Sv. G. 1978. Baltic amber, a palaeobiological study. Entomonograph 1: 1-92.
Lehmkuhl, D. M. 1979. How to know the aquatic insects. Wm. C. Brown Company Publishers, Dubuque, Iowa. 168 pp.
McCafferty, W. P. 1983. Aquatic entomology. Jones and Bartlett Publishers, Boston. 448 pp.
Nel, A. 1988. Les Sialidae (Megaloptera) fossiles des diatomites de Murat (Cantal, France) et de Bes-Konak (Anatolie, Turque). Neur. Int. 5: 39-44.
Neunzig, H. H., and J. R. Baker. 1991. Order Megaloptera, pp. 112-122 In F. W. Stehr (ed.). Immature insects, Vol. 2. Kendall/Hunt Publishing Company, Dubuque, Iowa. 975 pp.
New, T. R., and G. Theischinger. 1993. Megaloptera (Alderflies, Dobsonflies). Handbuch der Zoologie, Vol. 4 (Part 33). Walter de Gruyter, Berlin. 97 pp.
Parfin, S. I. 1952. The Megaloptera and Neuroptera of Minnesota. The American Midland Naturalist 47: 421-434.
Peckarsky, B. L., P. R. Fraissinet, M. A. Penton, and D. J. Conklin, Jr. 1990. Freshwater macroinvertebrates of northeastern North America. Cornell University Press, Ithaca. 442 pp.
Pennak, R. W. 1978. Fresh-water invertebrates of the United States, 2nd edition. John Wiley & Sons, New York. 803 pp.
Penny, N. D. 1977. Lista de Megaloptera, Neuroptera e Raphidioptera do México, América Central, ilhas Caraíbas e América do Sul. Acta Amazonica 7(4): Suplemento, 61 pp.
Penny, N. D. 1993. The phylogenetic position of Chloroniella peringueyi (Megaloptera: Corydalidae) and its zoogeographic significance. Ent. News 104: 17-30.
Pittman, J. L., T. S. Turner, L. Frederick, R. L. Petersen, M. E. Poston, M. Mackenzie, and R. M. Duffield. 1996. Occurrence of alderfly larvae (Megaloptera) in a West Virginia population of the purple pitcher plant, Sarracenia purpurea L. (Sarraceniaceae). Entomological News 107: 137-140.
Ponomarenko, A. G. 1976. Corydalidae (Megaloptera) from the Cretaceous of northern Asia. Ent. Rev. 55: 114-122.
Ponomarenko, A. G. 1977. Palaeozoic members of the Megaloptera (Insecta). Palaeont. J. [Washington]: 11: 73-81.
Pritchard, G., and T. G. Leischner. 1973. The life history and feeding habits of Sialis cornuta Ross in a series of abandoned beaver ponds (Insecta; Megaloptera). Can. J. Zool. 51: 121-131.
Riek, E. F. 1954. The Australian Megaloptera or alderflies. Aust. J. Zool. 2: 131-142.
Riek, E. F. 1974. Upper Triassic insects from the Molteno 'Formation', South Africa. Palaeontol. afr. 17: 19-31.
Smith, E. L. 1970. Biology and structure of the dobsonfly, Neohermes californicus (Walker) (Megaloptera: Corydalidae). The Pan-Pacific Entomologist 46: 142-150.
Stewart, K. W., G. P. Friday, and R. E. Rhame. 1973. Food habits of hellgrammite larvae, Corydalus cornutus (Megaloptera: Corydalidae), in the Brazos River, Texas. Ann. Entomol. Soc. Am. 66: 959-963.
Theischinger, G. 1983. The adults of the Australian Megaloptera. Aquatic Insects 5: 77-98.
Theischinger, G. 1991. Megaloptera (alderflies, dobsonflies), pp. 516-520 In C.S.I.R.O. (ed.). The insects of Australia, Vol. I. Cornell University Press, Ithaca. 542 pp.
Theischinger, G., and W. W. K. Houston. 1988. Megaloptera, pp. 23-32 In W. W. K. Houston (ed.). Zoological Catalogue of Australia, Vol. 6. Australian Government Publishing Service, Canberra.
Weele, H. W., van der. 1910. Megaloptera (Latreille), monographic revision, In Collections Zoologiques du Baron Edm. de Selys Longchamps Fasc. V (Première partie), Bruxelles. 93 pp.
Whiting, M. F. 1994. Cladistic analysis of the alderflies of America north of Mexico (Megaloptera: Sialidae). Systematic Entomology 19: 77-91.
Information on the Internet
- The Alderflies and Dobsonflies (Megaloptera) of South Africa. Compiled by Mervyn W. Mansell.
- Megaloptera Collection Holdings. California Academy of Sciences.
- Megaloptera Bibliography. North Carolina State University.
- Introduction to Megaloptera (CSIRO)
- Notes on the Neuroptera and Megaloptera of Madagascar and Adjacent Islands
- Key to the Hellgrammittes of California. Matthew Cover, UC Berkeley.
Title Illustrations

| Scientific Name | Nigronia sp. |
|---|---|
| Location | Abbeville County, South Carolina, USA |
| Specimen Condition | Live Specimen |
| Source | dark fishfly |
| Source Collection | Flickr |
| Image Use |
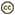 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2008 kim fleming |
| Scientific Name | Corydalus peruvianus |
|---|---|
| Location | Costa Rica |
| Sex | Female |
| Image Use |
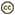 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0.
|
| Copyright |
© 1997 Ralph W. Holzenthal

|
| Scientific Name | Sialis salutary |
|---|---|
| Location | Flecknoe, Warwickshire, UK |
| Specimen Condition | Live Specimen |
| Source | flecknoe 09052008-17 |
| Source Collection | Flickr |
| Image Use |
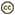 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2008 Walwyn |
About This Page

Universidad National Autónoma de México
Correspondence regarding this page should be directed to Atilano Contreras-Ramos at
Page copyright © 1997
 Page: Tree of Life
Megaloptera. Alderflies, dobsonflies, fishflies.
Authored by
Atilano Contreras-Ramos.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Megaloptera. Alderflies, dobsonflies, fishflies.
Authored by
Atilano Contreras-Ramos.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 12 November 1997
- Content changed 14 October 1997
Citing this page:
Contreras-Ramos, Atilano. 1997. Megaloptera. Alderflies, dobsonflies, fishflies. Version 14 October 1997. http://tolweb.org/Megaloptera/8218/1997.10.14 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site