Life cycle of Austramphilina elongata


Figure 1. Life cycle of Austramphilina elongata.
Adults (several cm long even when contracted, of yellow colour, Fig. 2) live in the body cavity of the long-necked turtle, Chelodina longicollis, in Southeastern Australia, but other species may be infected as well (Rohde, 1994, further references therein).

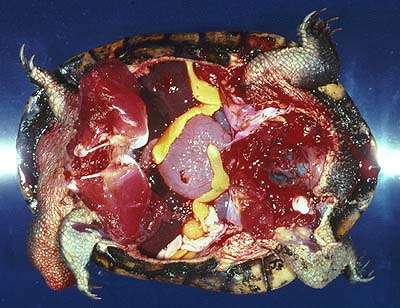
Figure 2. Adult Austramphilina elongata in the body cavity of a long-necked turtle.

Figure 3. Section through the lung of a long-necked turtle containing one adult Austramphilina elongata (arrow).
In one instance (among hundreds of turtles examined), an adult worm was found in the cloaca, suggesting that eggs may perhaps be laid in the cloaca and escape through the anus. However, adult worms were found repeatedly in the lungs (Fig. 3), and this suggests that eggs are laid in the lungs, enter the bronchioles and are carried in the trachea to the mouth cavity, from where they either escape in the sputum or are swallowed and leave the turtle in the faeces. Penetration of worms into the lungs is facilitated by a secretion which has very potent histolytic qualities.
Eggs are round and ciliated larvae hatch from them in freshwater. They swim around and actively penetrate into juvenile crayfish (Cherax destructor ) and freshwater shrimps (Parataya australiensis and Atya sp.). Only in the crayfish did they develop to the stage infective to turtles. It is likely that other species of crayfish can be infected as well. During penetration, larvae attach themselves to the cuticle of the host in a U-like fashion, i.e., the anterior and posterior ends are applied to the same small surface area. A secretion dissolving the cuticle is released at the anterior end, and the hooks at the posterior end hold the larva in position and saw through the cuticle. The hooks that hold the larva in position and possibly tear the cuticle apart have sharply pointed ends, while the hooks that do the sawing are serrate. The larva penetrates through the cleft thus produced with its anterior end first, leaving the ciliated epidermis behind. Successful penetration was observed to occur through the cuticle between the abdominal segments, and through the cuticle of the gills, where it is particularly thin. The larvae grow slowly and reach a size of several mm after several months, when they are infective to turtles. Infective stages were found almost exclusively in the muscles of the posterior part of the abdomen, suggesting that larvae migrate there or that only larvae that invade this part of the body survive.
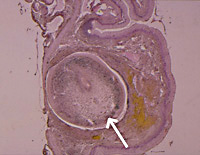
Figure 4. Section through the oesophagus of a long-necked turtle with a juvenile Austramphilina elongata (arrow) penetrating through it.
Turtles become infected when they eat infected crayfish. In experimental infections, juvenile worms were found to penetrate through the wall of the oesophagus (Fig. 4). They migrate along the trachea into the body cavity, where they grow and mature (Fig. 5) (Rohde, 1994, further references therein).

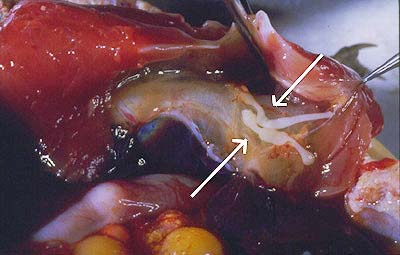
Figure 5. Juvenile Austramphilina elongata (arrows) migrating along trachea and oesophagus of long-necked turtle.
Life cycles of other species
Adult Amphilina foliacea live in the body cavity of European sturgeon, Acipenser. Eggs escape through the coelomic pore which connects the body cavity to the outside. Eggs containing infective larvae are ingested by freshwater amphipods. Chewing movements of the mouth parts of the crustaceans break the egg shell and enable the larvae to escape and penetrate into the host. Sturgeon become infected by eating infected amphipods. Adult Nesolecithus africanus live in African freshwater fish. Juvenile worms were recovered from freshwater prawns, Desmocaris trispinosa, in Nigeria (references in Rohde, 1994).
Intermediate hosts of all three species of amphilinids examined are crustaceans. It seems likely that other species have crustacean hosts as well.


 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site