Acantheae
Lucinda A. McDade and Carrie Kiel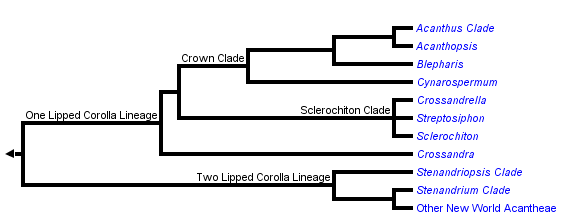

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Acantheae are well supported as the basal lineage of core Acanthaceae (i.e., those plants with retinaculate fruits) and also as a clade. The group has about 500 species that are currently placed in about 20 genera (but this taxonomy does not entirely reflect our recent phylogenetic results: McDade et al. 2005). Plants belonging to this lineage occur in both the New and Old Worlds; in the Old World, the vast majority of species occur in Africa with just a few in southern Europe and in Asia.
Characteristics
Among Acanthaceae, Acantheae are marked by the synapomorphy of monothecate anthers. A few other distantly related lineages of Acanthaceae have monothecate stamens (e.g., core Isoglossinae of Justicieae, McDade et al. 2000a, Kiel et al. 2006) but, in these lineages, the loss of one theca per stamen seems to have occurred after reduction to two stamens. In contrast, Acantheae retain the plesiomorphic trait of four stamens, and this combination of stamen (four) and thecae (one) number is, to our knowledge, unique among Acanthaceae.


Note the four stamens, each with a single theca or anther sac. This combination of traits is synapomorphic for Acantheae. © Lucinda McDade
Most New World Acantheae, members of the Stenandriopsis clade, Crossandra, Sclerochiton, and Streptosiphon have a five-lobed calyx with at least slightly unequal segments: the dorsal lobe is widest, the paired anterior lobes are narrower, and the paired lateral lobes are narrowest. We have proposed that the unequal, 1,2,2 configuration of the calyx is a synapomorphy for Acantheae (McDade et al. 2005). The trait has been further modified in the members of the one-lipped clade that have a four-lobed calyx (see Fig. 2g in McDade et al. 2005), and also in at least some members of the Stenandrium clade that have narrow, apparently equal segments. At least the basal members of the other main lineages of Acanthaceae s.s. have a calyx of five equal lobes, although there are further modifications in some lineages, notably Barlerieae.
As the phylogeny above suggests, the two main lineages of Acantheae can be characterized as having a plesiomorphic two-lipped corolla or a synapomorphic one-lipped corolla. As is typical of Acanthaceae and Lamiales in general, two-lipped corollas have two lobes in the upper lip and three lobes in the lower lip. Plants in the one-lipped clade have all five lobes of the corolla directed ventrally such that the plants lack an upper lip (although in some, the dorsal sepal seems to fulfill that role functionally).
References
Benoist, R. 1930. Descriptons d’espèces nouvelles d’Acanthacées de Madagascar. Bulletin de la Société botanique de France 76: 1031-1038.
Benoist, R. 1967. Acanthacées. In H. Humbert, Flore de Madagascar et des Comores 182, tome 1: 1-230.
Bentham, G. and J. D. Hooker. 1876. Genera Plantarum 2: 1060-1122. London: Reeve.
Daniel, T. F. 1983. Systematics of Holographis (Acanthaceae). Journal of the Arnold Arboretum 64: 129-160.
Daniel, T. F. 1985. A revision of Stenandrium (Acanthaceae) in Mexico and adjacent regions. Annals of the Missouri Botanical Garden 71: 1028-1043.
Daniel, T. F. 1991. A revision of Aphelandra (Acanthaceae) in Mexico. Proceedings of the California Academy of Sciences 47: 235-274.
Daniel, T. F. 2000. Additional chromosome numbers of American Acanthaceae. Systematic Botany 25: 15-25.
Daniel, T. F. and T. I. Chuang. 1998. Chromosome numbers of cultivated Acanthaceae and systematic implications. Pp. 309--330 in Diversity and taxonomy of tropical flowering plants, eds. P. Mathew and M. Sivadasan. Calicut, India: Mentor Books.
Daniel, T. F., T. I. Chuang, and M. A. Baker. 1990. Chromosome numbers of American Acanthaceae. Systematic Botany 15: 13-25.
Furness, C. A. 1990. Pollen morphology of Crossandra and Crossandrella (Acanthaceae: Acantheae). Grana 29: 161-176.
Furness, C. A. 1994. The caveate pollen of Streptosiphon hirsutus (Acanthaceae: Acantheae) and its taxonomic significance. Kew Bulletin 49: 409-414.
Grisebach, A. 1858. Novitiae florae panamensis. Bonplandia 6: 2-12.
Grisebach, A. H. R. 1864. Flora of the British West Indian Islands. London: Reeve and Co.
Heine, H. 1966. Acanthacées. Vol. 13 in Flore de Gabon, A. Aubréville (ed.). Paris: Muséum National D’Histoire Naturelle.
Hooker, W. J. 1837. Icones Plantarum, Volume 2. London: Longman, Rees, Orme, Brown, Green and Longman.
Hooker, W. J. 1845. Curtis’s botanical magazine, Volume 71. London: Reeve Bros.
Leonard, E. C. 1953. The Acanthaceae of Colombia, II. Contributions from the United States National Herbarium 31: 119-322.
Mangenot, S. and G. Mangenot. 1962. Enquête sur les nombres chromosomiques dans une collection d'espèces tropicales. Revue de Cytologie et de Biologie Végetales 25: 411-447.
Manktelow, M. 1996. Phaulopsis (Acanthaceae)—a monograph. Symbolae Botanicae Upsalienses 31: 1-184.
Manktelow, M., L. A. McDade, B. Oxelman, C. A. Furness, and M.-J. Balkwill. 2001. The enigmatic tribe Whitfieldieae (Acanthaceae): delimitation and phylogenetic relationships based on molecular and morphological data. Systematic Botany 26: 104-119.
McDade, L. A. 1982. Three new species of Aphelandra (Acanthaceae) from Central America. Annals of the Missouri Botanical Garden 69: 402-411.
McDade, L. A. 1984. Systematics and reproductive biology of the Central American species of the Aphelandra pulcherrima complex (Acanthaceae). Annals of the Missouri Botanical Garden 71: 104-165.
McDade, L. A. 1992. Pollinator relationships, biogeography, and phylogenetics. BioScience 42: 21-26.
McDade, L. A. and M. L. Moody. 1999. Phylogenetic relationships among Acanthaceae: evidence from non-coding trnL-trnF chloroplast DNA sequences. American Journal of Botany 86: 70-80.
McDade, L. A. and M. D. Turner. 1997. Extrafloral nectaries in Aphelandra: anatomy, development and systematic implications. American Journal of Botany 84: 1 15.
McDade, L. A., T. F. Daniel, S. E. Masta, and K. M. Riley. 2000a. Phylogenetic relationships within the tribe Justicieae (Acanthaceae): evidence from molecular sequences, morphology, and cytology. Annals of the Missouri Botanical Garden 87: 435-458.
McDade, L. A., S. E. Masta, M. L. Moody, and E. Waters. 2000b. Phylogenetic relationships among Acanthaceae: evidence from two genomes. Systematic Botany 25: 105-120.
McDade, L. A., T. F. Daniel, C. A. Kiel, and K. Vollesen. 2005. Phylogenetic relatinships among Acantheae (Acanthaceae): Major lineages present contrasting patterns of molecular evolution and morphological differentiation. Systematic Botany 30: 834-862.
Moylan, E. C., J. R. Bennett, M. A. Carine, R. G. Olmstead, and R. W. Scotland. 2004. Phylogenetic relationships among Strobilanthes s.l. (Acanthaceae): evidence from ITS nrDNA, trnL-F cpDNA, and morphology. American Journal of Botany 91: 724-735.
Schwarzbach, A. E. and L. A. McDade. 2002. Phylogenetic relationships of the mangrove family Avicenniaceae based on chloroplast and nuclear ribosomal DNA sequences. Systematic Botany 27: 84-98.
Scotland, R.W. and K. Vollesen. 2000. Classification of Acanthaceae. Kew Bulletin 55: 513-589.
Scotland, R.W., J. A. Sweere, P. A. Reeves, and R. G. Olmstead. 1995. Higher-level systematics of Acanthaceae determined by chloroplast DNA sequences. American Journal of Botany 82: 266-275.
Swofford, D. L. 2000. PAUP*. Phylogenetic analysis using parsimony (*And Other Methods). Version 4. Sunderland: Sinauer Associates.
Taberlet, P., L. Gielly, G. Pautou, and J. Bouvet. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105-1109.
Tomlinson, P. B. 1986. The botany of mangroves. Cambridge: Cambridge University Press.
Vollesen, K. 1990a. Notes on Crossandra (Acanthaceae). Kew Bulletin 45: 121-135.
Vollesen, K. 1990b. The genus Crossandra (Acanthaceae) in the African continent. Kew Bulletin 45: 503-534.
Vollesen, K. 1991. A revision of the African genus Sclerochiton (Acanthaceae: Acantheae). Kew Bulletin 46: 1-50.
Vollesen, K. 1992. The Old World species of Stenandrium (Acanthaceae: Acantheae). Kew Bulletin 47: 169-202.
Vollesen, K. 1994. The genus Streptosiphon (Acanthaceae: Acantheae). Kew Bulletin 9: 401-407.
Vollesen, K. 1997. Synopsis of the genus Crossandra (Acanthaceae) in Madagascar. Kew Bulletin 52: 379-415.
Vollesen, K. 1999. Cynarospermum, a new genus of Acanthaceae from India. Kew Bulletin 54: 171-177.
Vollesen, K. 2000. Blepharis (Acanthaceae): a taxonomic revision. Kew: Royal Botanic Gardens.
Wasshausen, D. C. 1975. The genus Aphelandra (Acanthaceae). Smithsonian Contributions to Botany no. 18.
Title Illustrations

| Scientific Name | Aphelandra impressa Lindau (Acantheae) |
|---|---|
| Location | Venezuela |
| Specimen Condition | Live Specimen |
| Identified By | L. A. McDade |
| Copyright | © 2006 Mark Skinner |
| Scientific Name | Blepharis Juss. |
|---|---|
| Location | Davenham, South Africa |
| Specimen Condition | Live Specimen |
| Collection | J |
| Copyright |
© 2006

|
| Scientific Name | Encephalosphaera lasiandra Mildbr. |
|---|---|
| Location | Peru |
| Comments | Image is on a herbarium at F |
| Specimen Condition | Live Specimen |
| Collector | J. G. Graham & J. Schunke Vigo |
| Copyright | © 2006 |
About This Page

Rancho Santa Ana Botanic Garden, Claremont, California, USA
Carrie Kiel

Rancho Santa Ana Botanic Garden
Correspondence regarding this page should be directed to Lucinda A. McDade at and Carrie Kiel at
Page copyright © 2009 and Carrie Kiel
All Rights Reserved.
- First online 12 September 2006
- Content changed 12 September 2006
Citing this page:
McDade, Lucinda A. and Carrie Kiel. 2006. Acantheae. Version 12 September 2006 (under construction). http://tolweb.org/Acantheae/52300/2006.09.12 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site