Evanioidea
Andrew R. Deans and John T. Jennings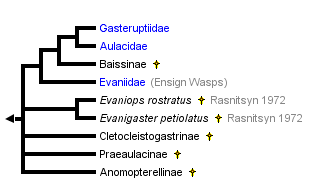

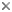
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Evanioidea comprises three extant families, Aulacidae, Gasteruptiidae, and Evaniidae, and several fossil taxa of uncertain placement. Some systematists have uncovered morphological (Naumann 1991, Whitfield et al. 1989, Gauld and Bolton 1988, Gibson 1985, Carlson 1979, Crosskey 1951), biological (Gauld and Bolton 1988, Crosskey 1951, Bradley 1908), and molecular (Dowton and Austin 2001, Dowton et al. 1997) evidence questioning the monophyly of Evanioidea, but no formal higher level taxonomic alterations have been made since Hedicke (1939).
Characteristics
Evanioidea share two possible apomorphies: the dorsal articulation of the metasoma to the mesosoma (Fig. 1) and the loss of all functional metasomal spiracles (breathing holes) except on the seventh segment (Figs. 1 and 2).

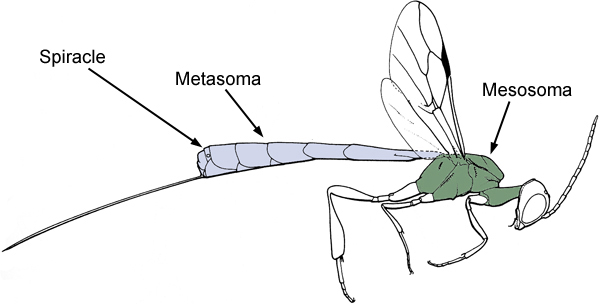
Figure 1. Diagram of a female Evanioid (Gasteruptiidae) showing the dorsal articulation of the metasoma to the mesosoma (after Goulet and Huber 1993).
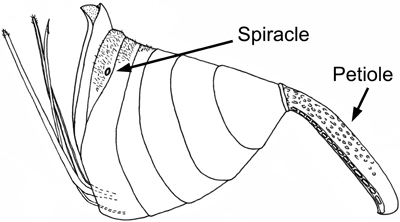
Figure 2. Evanioid (Evaniidae) metasoma (female) showing reduction of spiracles except for on the seventh segment. Image copyright © 2004 Andrew R. Deans.
Are Evanioidea monophyletic?
The most apparent evidence suggesting a non-monophyletic Evanioidea are the differences in host biology for the three families. Evaniids oviposit into cockroach oothecae buried in substrate, loose in leaf litter, or attached to female cockroaches. Gasteruptiids are predator-inquilines that lay eggs inside the cells of solitary bees and wasps nesting in plant stems or in underground nests, with the subsequent larvae developing on the food stores and/or nest inhabitants (Jennings and Austin 2004). Aulacids, considered by some hymenopterists to be the most ancestral of the three families, are endoparasitic on wood-boring xiphydriid sawflies and cerambycid and buprestid beetles (Jennings and Austin 2003). Many hymenopterists hypothesize an easy transition from parasitizing wood-boring larval hosts to "parasitizing" nest cells within plant stems but not to "parasitizing" cockroach egg cases (Whitfield 1998). Gasteruptiidae and Aulacidae also share more synapomorphies with each other than with Evaniidae and have frequently been combined into one family (Gasteruptiidae; see Townes 1950). These facts, combined with evidence that species in several other distantly related lineages (Chalcidoidea, Cynipoidea, Ichneumonoidea) independently evolved dorsally articulated metasomas, have led people to question the monophyly of Evanioidea.
Discussion of Phylogenetic Relationships
Despite conflicting evidence several recent phylogenetic and other studies predict an Evaniidae + Gasteruptiidae clade. Ronquist et al. (1999), using morphological data, recovered these two families as sister taxa with low support. Quicke et al. (1994) found some similarities between the ovipositor in Aulacidae and Gasteruptiidae, but not Evaniidae. Both families have a medial thickening of the ventral wall of the upper valve, but the Gasteruptiidae have a mid-dorsal longitudinal ridge that is absent in aulacids. Evaniidae differ in that their ovipositor is dorso-ventrally compressed rather than diverging, a character that may be functionally linked to parasitising blattodean oothecae.
Dowton and Austin (2001) recovered Evaniidae + Gasteruptiidae using evidence from three genes (Fig. 5 in Dowton and Austin 2001) and three genes plus morphological characters (Figs. 7,9,13 in Dowton and Austin 2001). However, their placement of Evaniidae depended on the tree building methods and models employed. Sister-group relationships also resulted between ensign wasps (Evaniidae) and other taxa, mainly Evaniidae + Heloridae (Figs. 2,3,6 in Dowton and Austin 2001) and Evaniidae + Ceraphronoidea (Figs. 4,10 in Dowton and Austin 2001). Unpublished reanalyses using Dowton and Austin's data (Deans and Whitfield 2003, using Bayesian methods) recovered a monophyletic Evanioidea 100% of the time, but the relationships between families differed depending on the assumptions made.
Fossil History
Deans et al. (2004) and Mason (1993) noted that several amber fossils (Fig. 3) reveal intermediate forms between Aulacidae and Evaniidae, lending credit to the idea that Evanioidea are monophyletic, and Jennings et al. (2004) noted that Hyptiogastrites electrinus Cockerell from Burmese amber (Fig. 4) exhibits characteristics of both Gasteruptiidae and Aulacidae.

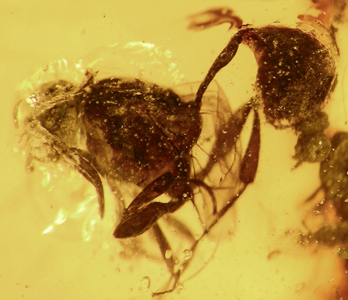
Figure 3. Evaniid fossil in Lebanese amber with some characteristics of the family Aulacidae. Image copyright © 2004 Andrew R. Deans.

Figure 4. Hyptiogastrites electrinus Cockerell in Burmese amber exhibiting characteristics of both Aulacidae and Gasteruptiidae. Image copyright © 2004 John T. Jennings.
Basibuyuk et al. (2002), Jennings et al. (2004), and Nel et al. (2004) provide excellent summaries of Evanioidea in the the fossil record. The phylogenetic structure at the top of the page was estimated by Basibuyuk et al. (2002). The stem group taxa (Evaniops, Evanigaster, Cletoclistogastrinae, Praeaulacinae, and Anomopterellinae) all belong to the fossil family Praeaulacidae, which are found in the Jurassic and Lower Cretaceous of Kazakhstan, East Siberia, Mongolia and Australia (Rasnitsyn 1972). Baissinae (at least 30 spp.; includes Manlayinae) has variably been placed in Gasteruptiidae, Aulacidae, or its own family (Baissidae) (Nel et al. 2004), though the latest estimation of relationships places Baissinae sister to extant Aulacidae and Gasteruptiidae (Basibuyuk et al. 2002).
Evanioidea are represented by close to 100 fossil species. Our understanding of how these taxa relate to extant groups remains unstable due to the incomplete preservation of most fossils and the lack of phylogenetic characters therein. Future efforts in this research area are needed and will undoubtedly shed light on the evolution of this group of insects.
References
Basibuyuk, H.H., Rasnitsyn, A.P., Fitton, M.G., Quicke, D.L.J. 2002. The limits of the family Evaniidae (Insecta:
Hymenoptera) and a new genus from Lebanese amber. Insect Systematics and Evolution 33: 23-34.
Bradley, J. C. 1908. The Evaniidae, ensign flies, an archaic family of Hymenoptera. Transactions of the American Entomological Society 34: 101-194.
Carlson, R. W. 1979. Superfamily Evanioidea. Pp. 1109-1118 in: Catalog of the Hymenoptera in America North of Mexico, volume 1, Symphyta and Apocrita (Parasitica). K. V. Krombein, P. D. Hurd, D. R. Smith, and B. D. Burks, eds. Smithsonian Institution Press, Washington, D. C., U.S.A. 1198 pp.
Crosskey, R. W. 1951. The morphology, taxonomy, and biology of the British Evanioidea (Hymenoptera). Transactions of the Royal Entomological Society of London 102: 247-281.
Deans, A. R., H. H. Basibuyuk, D. Azar, and A. Nel. 2004. Descriptions of two new Early Cretaceous (Hauterivian) ensign wasp genera (Hymenoptera: Evaniidae) from Lebanese amber. Cretaceous Research 25: 509-516.
Deans, A. R. and J. B. Whitfield. 2003. Estimates of relationships between ensign wasp genera (Hymenoptera: Evaniidae). Evolution Conference 2004. Fort Collins, CO. June 26-30, 2004.
Dowton, M. and A. D. Austin. 2001. Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita - evolutionary transitions among parasitic wasps. Biological Journal of the Linnaean Society 74: 87-111.
Dowton, M., A.D. Austin, N. Dillon, and E. Bartowsky. 1997. Molecular phylogeny of the apocritan wasps with particular reference to the Proctotrupomorpha and Evaniomorpha. Systematic Entomology 22: 245-255.
Gauld, I. D. and B. Bolton. 1988. The Hymenoptera. Oxford University Press, Oxford, U.K. 332 pp.
Gibson, G. A. P. 1985. Some pro- and mesothoracic structures important for phylogenetic analysis of Hymenoptera, with a review of the terms used for the structures. Canadian Entomologist 117: 1395-1443.
Goulet, H. and J. T. Huber (eds.) 1993. Hymenoptera of the World: An Identification Guide to Families. Research Branch, Agriculture Canada Publication 1894/E. 668 pp.
Hedicke, H. 1939. Evaniidae. In: Hymenoptorum Catalogus, H. Hedicke (ed.) Pars 9. Dr. W. Junk. 's-Gravenhage, 50 pp.
Jennings, J. T. and A. D. Austin. 2004. Biology and host relationships of aulacid and gasteruptiid wasps (Hymenoptera: Aulacidae): a review. Pp. 187-215. In K. Rajmohana, K. Sudheer, P. Girish Kumar, and S. Santhosh (eds.) Perspectives on Biosystematics and Biodiversity. University of Calicut, Kerala, India.
Jennings, J. T., A. D. Austin, and N. B. Stevens. 2004. Hyptiogastrites electrinus Cockerell, 1917, from Burmese amber: redescription and its placement within the Evanioidea (Hymenoptera). Journal of Systematic Palaeontology 2: 127-132.
Mason, W. R. M. 1993. Superfamilies Evanioidea, Stephanoidea, Megalyroidea, and Trigonalyoidea. Chapter 11, pp. 510-520 In: Goulet, H. and J. T. Huber, eds. Hymenoptera of the World: An identification guide to families. Research Branch, Agriculture Canada, Publication 1894/E. 668 pp.
Naumann, I. D. 1991. Hymenoptera. Pp. 916-1000 In: The Insects of Australia, Volume II. Melbourne University Press, Melbourne, Australia.
Nel, A., A. Waller, and G. De Ploëg. 2004. An aulacid wasp in the Lowermost Eocene amber from the Paris Basin (Hymenoptera: Aulacidae). Geologica Acta 2 (1): 67-74.
Quicke, D. L. J., M. G. Fitton, J. R. Tunstead, S. N. Ingram, and P. V. Gaitens. 1994. Ovipositor structure and relationships within the Hymenoptera, with special reference to the Ichneumonoidea. Journal of Natural History 28: 635-682.
Rasnitsyn A.P. 1972. Praeaulacidae (Hymenoptera) from the Upper Jurassic of Karatau. Paleontologicheskiy Zhurnal 1: 72-87 [in Russian, translated into English as 'Late Jurassic Hymenopterous insects (Praeaulacidae) of Karatau)' in Paleontological Journal 6(1): 62-77]
Ronquist, F., A. P. Rasnitsyn, A. Roy, K. Eriksson, and M. Lindgren. 1999. Phylogeny of the Hymenoptera: a cladistic reanalysis of Rasnitsyn's (1988) data. Zoologica Scripta 28: 13-50.
Sharkey, M. J. and A. Roy. 2002. Phylogeny of the Hymenoptera: a reanalysis of the Ronquist et al. (1999) reranalysis, emphasizing wing venation and apocritan relationships. Zoologica Scripta 31: 57-66.
Townes, H. K. 1950. The Nearctic species of Gasteruptiidae (Hymenoptera). Proceedings of the United States National Museum 100: 85-145.
Whitfield, J. B. 1998. Phylogeny and evolution of host-parasitoid interactions in Hymenoptera. Annual Review of Entomology 43: 129-151.
Whitfield, J. B., N. F. Johnson, and M. R. Hamerski. 1989. Identity and phylogenetic significance of the metapostnotum in nonaculeate Hymenoptera. Annals of the Entomological Society of America 82: 663-673.
Title Illustrations

| Scientific Name | Aulacidae |
|---|---|
| Location | Australia |
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Life Cycle Stage | adult |
| Body Part | habitus |
| Size | ~12mm |
| Image Use |
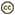 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0.
|
| Copyright |
© 2004 John T. Jennings

|
| Scientific Name | Evaniidae |
|---|---|
| Location | Honduras |
| Creator | Andrew. R. Deans |
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Life Cycle Stage | adult |
| Body Part | habitus |
| Size | ~11mm |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0.
|
| Copyright |
© 2004 Andrew R. Deans

|
About This Page
Andrew R. Deans

Department of Entomology, NC State University
John T. Jennings

University of Adelaide, Glen Osmond, South Australia, Australia
Correspondence regarding this page should be directed to Andrew R. Deans at and John T. Jennings at
Page copyright © 2004 Andrew R. Deans and John T. Jennings
 Page: Tree of Life
Evanioidea.
Authored by
Andrew R. Deans and John T. Jennings.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Evanioidea.
Authored by
Andrew R. Deans and John T. Jennings.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 04 October 2004
- Content changed 23 May 2006
Citing this page:
Deans, Andrew R. and John T. Jennings. 2006. Evanioidea. Version 23 May 2006. http://tolweb.org/Evanioidea/11170/2006.05.23 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site