Tetraodontiformes
triggerfishes, boxfishes, puffers (fugu), molas and allies
James C. Tyler and Nancy Holcroft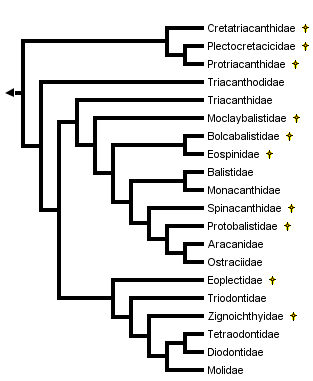

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxCladogram based on Santini and Tyler, 2003, as modified for the basal tetraodontoids by Tyler et al., 2006.
Introduction
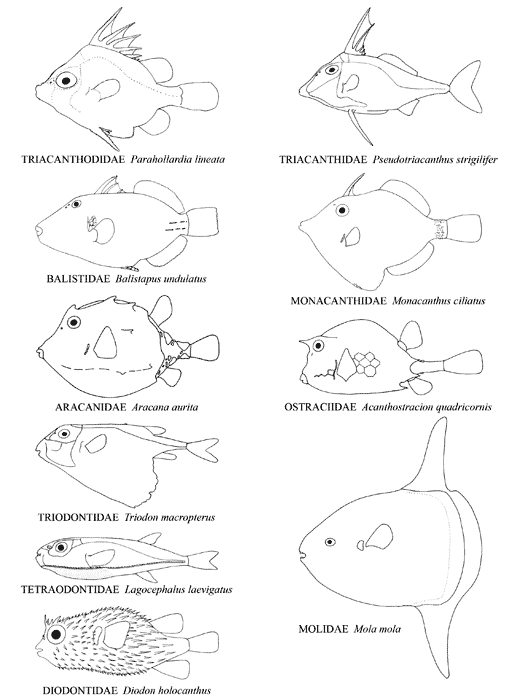
Body shape in representatives of the ten extant families of tetraodontiforms. Composit of figures from Tyler, 1980, ©
The Order Tetraodontiformes comprises approximately 350 species of highly derived acanthomorph fishes notable for their exceptional degree of diversity in morphological structure, shape, size, and way of life. They are found in temperate and tropical marine waters worldwide, although a few families are absent in the eastern Pacific and the Atlantic, and some species are found in the fresh waters of South America, Africa, and south-east Asia. They are grouped in ten well-defined extant families, each of whose monophyly is unequivocal; however, questions remain about how some of these families are interrelated. The families and their respective general habitats are as follows:
Triacanthodidae (spikefishes), deep-water, bottom-dwelling (one species bathypelagic), absent in the eastern Pacific, the most basal extant linage of tetraodontiforms;
Triacanthidae (triplespines), shallow continental bottom waters, absent from the Atlantic and the eastern Pacific;
Balistidae (triggerfishes) and its sister group Monacanthidae (filefishes), often associated with coral or rocky reefs and surrounding sand and sea grass beds, with a few pelagic species;
Aracanidae (boxfishes, absent from the Atlantic and the eastern Pacific) and its sister group Ostraciidae (trunkfishes), associated with coral and rocky reefs and surrounding shallow bottoms (some trunkfishes with pelagic juvenile stages; one trunkfish species matures and spawns pelagically);
Triodontidae (three-tooth puffers), a single extant species, in Indo-western Pacific deep bottom waters;
Tetraodontidae (puffers or fugu) and its sister group Diodontidae (porcupinefishes), associated with coral and rocky reefs and shallow bottoms, with a few pelagic species;
Molidae (ocean sunfishes), three well-known pelagic species in oceans worldwide, and perhaps one or two more localized regional taxa confined to southern oceans or Austral-Asia.
Characteristics
Description
Some of the easily seen distinguishing features of extant tetraodontiforms are as follows: anal-fin spines lacking; dorsal-fin spines six or fewer or absent; pelvic fin either reduced to a spine and no more than two small rays, or forming a bony rudimentary structure at the end of the pelvis, or absent entirely; scales on the body often thick and specialized as bony plates or as protruding spines; relatively small mouths (although the jaws are sometimes massive) with few and often enlarged teeth; a small gill opening restricted to the side of the head, the opening not extending much below the base of the pectoral fin; and caudal fin with 12 or fewer principal rays and usually no procurrent rays. The number of vertebrae is 20 (8+12) or less in the more basal clades of tetraodontiforms, but the number is secondarily increased in many taxa. Adult sizes range from minute (15–20 mm SL in the monacanthid Rudarius) to huge (up to about 3 meters in length and 2,200 kilograms in weight for Mola) (Tyler, 1968, 1980; Matsuura and Tyler, 1994; Hutchins, 2002; T. Thys, personal communication, September 2006).



Tetraodontiform fishes show great variation in body size: Ocean sunfish, Mola mola, (image © ), can be up to 3 meters long, while the minute leatherjacket, Rudarius minutus (© 2006 Nick Hobgood), only reaches a size of a few centimeters.
The hallmark of the evolutionary diversification of the tetraodontiforms is that it has been achieved mainly through processes of reduction, simplification, and loss of morphological elements, especially of the internal bony parts. Such reductive trends are not uncommon in other groups of fishes, but it is of special interest in tetraodontiforms because of the extreme exaggeration of the tendency in the order. Of course, reductive trends are not absolute in tetraodontiforms, and certain morphological systems in circumscribed groups show opposite tendencies, such as the size and complexity of the jaws and of the stomach and its complex musculature in puffers and porcupinefishes, the pectoral girdle of boxfishes and trunkfishes, and the scales in many groups (Tyler, 1962, 1963, 1980; Lauder and Liem, 1983; Tyler and Sorbini, 1996). The reductive trend even extends to the molecular level; tetraodontids have the smallest genomes among vertebrates measured to date, and a reduction in genome size appears to have evolved independently in some balistoids as well (Brainerd et al., 2001, and contained references).
List of Synapomorphies of the Tetraodontiformes
The following are synapomorphies for tetraodontiforms, both fossil and extant (Santini and Tyler 2003):
- medial edges of pterosphenoids not in contact and well separated;
- supraocular serrations of frontal absent;
- nasal absent;
- posterior crest of supraoccipital absent;
- parietal absent;
- basisphenoid absent;
- postmaxillary process absent;
- reduced or absent ornamentation of skull bones and lack of spiny processes;
- elongate ceratohyal;
- ceratohyal without a deep dorsal concavity;
- fifth ceratobranchial without gill rakers along anterior edge;
- gill opening small and slit-like;
- posterior pelvic process fused or highly consolidated;
- posterior pelvic process long, tapering, shaft-like, roundish in cross section;
- ribs absent;
- haemal spine of PU3 non-autogenous;
- spiny dorsal-fin locking mechanism present;
- two spiny dorsal-fin pterygiophores anterior to fourth neural spine;
- anal-fin spine absent;
- one epural;
- hypurapophysis absent;
- supratemporal scale bones absent;
- no sheath of scaly skin along soft dorsal fin;
- one or two pelvic-fin rays;
- eight or nine abdominal vertebrae;
- six dorsal-fin spines;
- five spiny dorsal-fin pterygiophores;
- ten to fourteen soft dorsal-fin rays.
The following are synapomorphies for the extant tetraodontiforms and for the fossils of Paleocene age or younger (i.e., excluding the most basal tetraodontiform clade, the Upper Cretaceous Plectocretacicoidea, a three-family clade that is the sister group of all of the other clades):
- infraorbitals absent;
- dentary groove for sensory canal absent;
- mouth gap moderate to small;
- upper jaw only slightly protractile;
- skull bone canals for lateral line system absent;
- thick caniniform teeth;
- teeth internal to major outer series in upper jaw present;
- teeth internal to major outer series in lower jaw present;
- PU2 first caudal peduncle vertebra with modified neural spine;
- PU2 first caudal peduncle vertebra with modified haemal spine;
- second dorsal-fin spine long;
- first anal pterygiophore along rear edge of first caudal haemal spine;
- one anal pterygiophore in first interhaemal space;
- three or fewer anal pterygiophores anterior to third caudal haemal spine;
- five caudal peduncle vertebrae;
- procurrent caudal-fin rays absent;
- scales with thin basal plates bearing upright spinules;
- no sheath of scaly skin along anal fin;
- ten to fourteen anal-fin rays.
Discussion of Phylogenetic Relationships
Because tetraodontiforms often have bizarre appearances or behavior, and representatives of many of the major subgroups occur in shallow water in the Mediterranean Sea and along the Atlantic coast of Europe, they were well known to ancient cultures, from the Egyptians to the Greeks and Romans. At the birth of systematics, they already were recognized as a natural group by Linnaeus and were first given formal ordinal status (as the Plectognathi) by Cuvier (1817) in Le Règne Animal. Many different interpretations of supposed relationships of the families have been proposed since then; this history was discussed in detail by Tyler (1980). Suffice it to say that, following Cuvier and until recent decades, the tetraodontiforms were recognized as belonging to two main groups or lineages: the scleroderms (triacanthoids, balistoids, ostracioids) for those with individual teeth protruding from sockets in the jaws and often with thick scales or carapace plates; and the gymnodonts (triodontids, tetraodontoids, molids) for those with the teeth highly modified and complexly incorporated into a parrot-like beak and with the scales modified, most often into prickly spines of some sort.
In recent decades, several workers have surveyed the morphological and molecular systems of tetraodontiforms and have used their resultant relatively large data sets to assess familial relationships and to try to determine the sister group of the order (see Santini and Tyler, 2003, and Holcroft, 2004, 2005 for more details on the following).
Morphological hypotheses
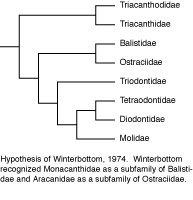
The first cladistic analysis of tetraodontiform interrelationships was that of Winterbottom (1974). His analysis was based upon a comprehensive study of the myology (75 muscles) of several representatives of each of the extant families (with the exception of Triodontidae, which contains only a single extant species; this exception also applies to all other authors’ analyses discussed below). He recognized three main clades: Triacanthodidae + Triacanthidae, as sister to all other extant clades; the latter comprising the scleroderm clade Balistidae + Monacanthidae as sister to Aracanidae + Ostraciidae; and the gymnodont clade, with Triodontidae as sister to (Tetraodontidae + Diodontidae) + Molidae.
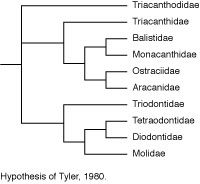
Tyler’s (1980) phylogenetic hypothesis was based upon the osteology of numerous extant representatives of each of the families and of most of the then known fossil taxa. The evolutionary taxonomic interpretation of Tyler was very similar to that of Winterbottom, with a clade of balistoids + ostracioids among the scleroderms and the same sequential clade of gymnodonts with triodontids as sister to (tetraodontids + diodontids) + molids. Tyler’s treatment of the triacanthoids, however, was a combination of primitive and advanced features, with an emphasis on the Eocene Eoplectidae (Eoplectus) being the ancestor of the gymnodonts even though it was placed in the triacanthoids because of its numerous plesiomorphic attributes.
The interpretations of both Winterbottom (1974) and Tyler (1968, 1980) were based upon the then-current assumption that tetraodontiforms were, in some ill-defined way, closely related to acanthuroids, as originally proposed by Cope (1871) and Dareste (1872). Winterbottom (1974) also stated that some zeiforms might be related to tetraodontiforms. In a study that focused on squamipinnes, Mok and Shen (1983) also concluded that acanthuroids (siganids, zanclids, acanthurids) were sister to tetraodontiforms, and Lauder and Liem (1983) also accepted that view.
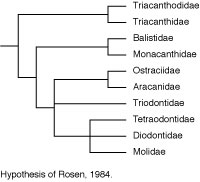
Rosen (1984) made a major departure from this accepted wisdom in his comparison of acanthuroids, caproids, zeiforms, and tetraodontiforms. He concluded that there are seven synapomorphies uniting caproids, zeiforms, and tetraodontiforms, and four synapomorphies uniting zeiforms and tetraodontiforms (see Tyler et al., 2003 for a detailed critical review of these features and interpretation). These putative relationships remain controversial because there is evidence that zeiforms are lower acanthomorphs, and perhaps not even percomorphs, whereas caproids are considered to be among the higher percoids (see Holcroft, 2004).
Tyler et al. (2003) provided a detailed account of the osteology of the zeiforms in an effort to access the strength of the putative zeiform relationship with tetraodontiforms. They conducted four different analyses (characters ordered versus unordered, and meristic characters included or mostly excluded) of their 103 characters, using seven acanthomorph outgroups. All four analyses strongly supported the monophyly of zeiforms, which were arranged in six families. The topologies resulting from three of the analyses supported a phylogeny in which caproids are the sister group of zeiforms + tetraodontiforms. The topology of the fourth analysis, however, with the characters unordered and without meristics, was largely unresolved outside of the zeiforms and neither supported nor rejected Rosen’s (1984) hypothesis of the zeiforms relationship with caproids and tetraodontiforms. This fourth cladogram was considered by Tyler et al. (2003) to be the most rational and conservative, with the least number of assumptions, because in ordering the data it was found that the assigned polarities were often incorrect, and because the meristics of zeiforms are especially variable. For example, the structure of the first pelvic-fin element varies from ray-like to spine-like within the two genera of one group, and the number of anal-fin spines can vary not only between taxa of the same family but also intraspecifically, whereas in most other groups of fishes such features are almost invariable.
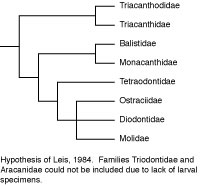
In another departure from the customary, Leis (1984) utilized 23 early life history or larval characteristics to cladistically analyze the relationships of those tetraodontiform families for which such data are available (not available for triodontids and aracanids, and incomplete for triacanthodids). Leis was aware of Rosen’s about-to-be-published work, so he used acanthuroids and zeiforms as alternate outgroups for tetraodontiforms. The resultant phylogeny recognized triacanthodids + triacanthids as the basal sister group to all other tetraodontiform families (as in Winterbottom, 1974). These higher clades, however, consisted of balistids + monacanthids as the sister group to a tetraodontoid clade comprised of the tetraodontids as sister to a three family, unresolved trichotomy of ostraciids + diodontids + molids. The trichotomy was supported by five early life history characters. This is the only cladistic analysis in which ostraciids (which have separate teeth protruding from sockets in the jaws) are not only closely associated with gymnodont fishes (which have specialized teeth complexly incorporated into beak-like jaws), they are also deeply nested within the gymnodonts. It is of interest that Britz and Johnson (2005) found an impressive additional early life history character supporting a sister-group relationship between ostraciids and molids (occipital-vertebral fusion in the ostraciid Lactophrys and in the molids Ranzania and Masturus). The putative relationship of ostraciids (and presumably also of aracanids, which was not included in Leis’ analysis due to lack of data, but which has been universally accepted as the sister group of ostraciids) with molids and other tetraodontoids requires further investigation with larger data sets encompassing a variety of types of data. For comparison with the early life history characters, we simply note that a far larger number of adult osteological characters support the sister-group relationship of fossil and extant balistoids and ostracioids in the phylogeny proposed by Santini and Tyler (2003), and that the molecular data on this issue are ambiguous (Holcroft, 2005).
At about the same time of Leis’ hypothesis, Winterbottom and Tyler (1983) presented a large number of osteological and myological synapomorphies that were based upon a reinterpretation of the data in Winterbottom (1974) and Tyler (1980) and that substantially supported the sister-group relationship of balistoids and ostracioids (although some errors in coding weakened the support). Klassen (1995) gave additional support for the balistoid + ostracioid clade. Matsuura (1979) provided an analysis of numerous osteological features of balistoids that strongly supported the sister-group relationship of balistids and monacanthids and that offered the most extensive phylogenetic hypotheses to date of the generic relationships within both families.
The osteological and other morphological data in the comparative diagnoses of the familial and higher categories in Tyler’s (1980) treatment of tetraodontiforms are essentially a list of contrasting character states differentiating the subgroups of the order. These characters were put into a cladistic format by Santini and Tyler (2002a, b) to produce hypotheses for the intrafamilial relationships of the extant species of, respectively, Triacanthidae and Molidae, but they did not attempt to determine the relationships of those two clades with other tetraodontiform lineages.
Santini and Tyler (2003) also utilized the osteological data given in the comparative diagnoses of tetraodontiform subgroups in Tyler (1980), as well as recently summarized data on all of the fossil taxa of the order as reviewed by Tyler and Santini (2002), for a phylogenetic hypothesis of the extant and fossil taxa of the order. At least two taxa representing each of the ten extant families, and all of the fossil taxa (Upper Cretaceous to Pliocene), were included in the analysis, with a total of 210 morphological characters for 20 extant and 36 fossil taxa of tetraodontiforms and two outgroups (a caproid and a zeiform). The analysis of this matrix for extant taxa alone produced two equally parsimonious trees that called into question the monophyly of some of the previously accepted major higher-level tetraodontiform clades (e.g., the strict consensus tree for exclusively extant taxa produced an unresolved trichotomy of balistoids, ostracioids, and tetraodontoids). The inclusion of the fossil taxa in the analysis, however, helped to resolve relationships between the extant family-level clades, as follows: Triacanthodidae are the sister group of the other nine extant families, which are grouped in two clades, the scleroderm families with Triacanthidae as sister to (Balistidae + Monacanthidae) + (Aracanidae + Ostraciidae), and the gymnodont families with Triodontidae as sister to (Tetraodontidae + Diodontidae) + Molidae. Regarding the fossil taxa, only two groups are of special interest to this discussion of the phylogeny of the extant families, with the Upper Cretaceous Plectocretacicoidea as sister to all other tetraodontiforms (Paleocene to Recent), and the Eoplectidae (Eocene of Monte Bolca) as the sister to all of the gymnodonts (i.e., Triodontidae and higher clades); see Tyler and Santini, 2002, for an illustrated review of the fossil tetraodontiforms.
The otoliths of fishes offer data sets that can be analyzed relatively independently of those that are based upon more traditional morphology. They can also be used to test analyses based upon traditional morphologies. Previously, little was known about the otoliths of tetraodontiforms, but a recently published comprehensive survey compared the otoliths of 87 species of extant tetraodontiforms, representing all 10 families, with those of a wide selection of 24 species of zeiforms, representing all six families, and with representative caproids and other acanthomorphs (Nolf and Tyler, 2006). That survey found several synapomorphies supporting the hypothesis of caproids as the sister group of zeiforms + tetraodontiforms. Within the tetraodontiforms, derived otolith structures were generally in agreement with the phylogeny proposed by Winterbottom (1974), Tyler (1980), and Santini and Tyler (2003), namely: triacanthodids and triacanthids being basal plesiomorphic families; balistids and monacanthids having a more derived structure, and the monophyly of monacanthids strongly supported; strong support for the monophyly of aracanids + ostraciids; triodontids having otoliths comparable with those of tetraodontoids; tetraodontids and diodontids united by a synapomorphy (even though the otoliths are of great diversity of derived structure); and molids (Ranzania) most similar to diodontids.
Molecular hypotheses
Several recent studies have attempted to use molecular data from both mitochondrial and nuclear genes to resolve some of the uncertainties regarding both tetraodontiform interrelationships and the placement of the order within a larger acanthomorph context. The latter has remained especially elusive to traditional morphological methods for more than 100 years. A number of large-scale molecular studies of teleost or acanthomorph relationships (Chen et al., 2003; Miya et al., 2003; Dettai and Lecointre, 2005) have included a limited number of tetraodontiform representatives. The results of these studies are somewhat surprising given previous morphological hypotheses regarding tetraodontiform affinities.
Chen et al. (2003) used data from the 12S and 16S mitochondrial genes and the nuclear genes 28S and rhodopsin to look for repeated clades within Acanthomorpha. Based upon these data for the included taxa, Tetraodontiformes could not be recovered as a monophyletic group (most likely because of problems associated with low taxon sampling), and the positions of the tetraodontiform representatives were unstable among the phylogenies they presented. Miya et al. (2003) analyzed mitogenomic sequences (including 12 protein-coding and 21 tRNA mitochondrial genes) for 100 species of higher teleosts, including two tetraodontiforms (Sufflamen fraenatus, a balistid, and Stephanolepis cirrhifer, a monacanthid). The analysis recovered the caproid Antigonia capros as the sister of the two tetraodontiforms with moderately high support. This caproid-tetraodontiform clade was sister to a monophyletic lophiiform clade, again with moderately high support. Whereas a relationship with Caproidae had been proposed previously based upon morphological data (i.e., Rosen, 1984), a close relationship between Tetraodontiformes and Lophiiformes had never been proposed as the result of a morphological analysis. Dettai and Lecointre’s (2005) analysis of two portions of the nuclear MLL gene partially corroborated the results of Miya et al. (2003). They included five tetraodontiform species representing four families (Triacanthodidae, Ostraciidae, Tetraodontidae, and Molidae); these were recovered as a monophyletic group in a weakly supported polytomy with Caproidae, Lophiiformes, a clade comprising Siganidae + Elassomatidae, and a clade comprising Pomacanthidae + Acanthuridae. This polytomy was recovered, with very weak support, as the sister to a chaetodontid + drepaneid clade.
Holcroft (2004) used data from the nuclear gene RAG1 to attempt to test the two primary alternative hypotheses regarding the tetraodontiform sister group (i.e., Zeiformes vs. Acanthuroidei). Her results directly supported neither hypothesis; the analysis of the RAG1 data recovered a monophyletic Tetraodontiformes as sister to an ephippid + drepaneid clade (the support for this relationship was very weak, however). Because of a general lack of support within the broad smegmamorph-percomorph clade in which the tetraodontiforms were nested, the results might best be interpreted as rejecting the hypothesis that zeiforms are the tetraodontiform sister group but leaving open the possibility that the tetraodontiforms share a close relationship with caproids and/or with some or all of the acanthuroids.
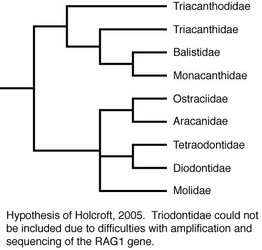
Molecular methods also have been utilized to examine relationships within the order. Holcroft (2005) used data from the nuclear gene RAG1 and the mitochondrial genes 12S and 16S to examine the interrelationships of 63 tetraodontiform species representing nine of the ten families (Triodontidae was excluded due to difficulties in amplifying and sequencing target gene regions). Holcroft considered the topologies resulting from the parsimony and Bayesian analyses of the RAG1 data alone to provide the best estimate of phylogeny based upon data collected for that study (for a discussion of the reasoning for this, see Holcroft, 2005). The RAG1 data recovered a strongly supported monophyletic Order Tetraodontiformes, and the monophyly of all families for which more than one representative was included (Balistidae, Monacanthidae, Aracanidae, Ostraciidae, Tetraodontidae, Diodontidae, Molidae) was corroborated with robust support as well. The parsimony and Bayesian topologies differed slightly, primarily with regard to placement of Triacanthodidae, Triacanthidae, Aracanidae + Ostraciidae, and Molidae. The parsimony analyses recovered two large, weakly supported clades within the order. The first comprised Triacanthodidae, Triacanthidae, Balistidae, and Monacanthidae; a sister-group relationship between Balistidae and Monacanthidae received robust support, with Triacanthodidae and Triacanthidae as very weakly supported sequential outgroups. Within the second clade, Aracanidae and Ostraciidae were recovered as sister groups with strong support, and this aracanid + ostraciid clade was placed in an unresolved polytomy with Molidae and a well-supported tetraodontid + diodontid clade. In the Bayesian analyses, three clades were recovered in an unresolved trichotomy. The first clade itself contained another unresolved trichotomy among Triacanthodidae, Triacanthidae, and a well-supported Balistidae + Monacanthidae clade. The second clade, which received weak support, comprised Molidae as the sister to Aracanidae + Ostraciidae. Within the third clade, Tetraodontidae and Diodontidae were recovered as sister groups with strong support. Using both parsimony and Bayesian methods, the RAG1 data also supported the monophyly of most tetraodontiform genera for which multiple representatives were included (the only exceptions were the monacanthid genus Cantherhines, in which Amanses was nested, and the balistid genus Balistoides, for which one representative was recovered as the sister of Xanthichthys and the other was the sister of a larger balistid clade).
It is clear from the above that much more remains to be discovered and understood about the phylogenetic interrelationships of the ten families of tetraodontiform fishes, and that the interpretation of some of these interrelationships are still in a state of flux. Future studies incorporating both fossil and extant taxa and data from both morphology and molecules will likely have the best chance at elucidating the evolutionary history of these bizarre and wonderful fishes.
References
Brainerd, E.L., Slutz, S.S., Hall, E.K., and Phillis, R.W. 2001. Patterns of genome size evolution in tetraodontiform fishes. Evolution, 55(11):23632368.
Britz, R. and Johnson, D.G. 2005. Occipito-vertebral fusion in ocean sunfishes (Teleostei: Tetraodontiformes: Molidae) and its phylogenetic implications. Journal of Morphology, 266:7479.
Chen, W.-J., Bonillo, C., and Lecointre, G. 2003. Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of Acanthomorpha (Teleostei) with larger number of taxa. Molecular Phylogenetics and Evolution, 26:262288.
Cope, E.D. 1871. Observations on the systematic relations of the fishes. American Naturalist, 5:579593.
Cuvier, G. 1817. Le Rθgne Animal. Vol. 2, 532 pp. Paris: Deterville.
Dareste, C. 1872. On the natural affinities of the Balistidae. Annales and Magazine of Natural History, ser. 4, 10:6870.
Dettai, A. and Lecointre, G. 2005. Further support for the clades obtained by multiple molecular phylogenies in the acanthomorph bush. Comptes Rendus Biologies, 328:674689.
Holcroft, N.I. 2004. A molecular test of alternative hypotheses of tetraodontiform (Acanthomorpha: Tetraodontiformes) sister group relationships using data from the RAG1 gene. Molecular Phylogenetics and Evolution, 32:749760.
Holcroft, N.I. 2005. A molecular analysis of the interrelationships of tetraodontiform fishes (Acanthomorpha: Tetraodontiformes). Molecular Phylogenetics and Evolution, 34:525544.
Hutchins, J.B. 2002. Description of a new genus and species of minute monacanthid fish from the Seychelles and Marshall Islands. Records of the Western Australian Museum, 21:213219.
Klassen, G.J. 1995. Phylogeny and biogeography of the Ostraciinae (Tetraodontiformes: Ostraciidae). Bulletin of Marine Science, 57(2):393441.
Lauder, G.V. and Liem, K.F. 1983. The evolution and interrelationships of the actinopterygian fishes. Bulletin of the Museum of Comparative Zoology, Harvard University, 150:95197.
Leis, J.M. 1984. Tetraodontiformes: relationships. In: Moser, H.G., edit. Ontogeny and systematics of fishes. American Society of Ichthyologists and Herpetologists, special publication. Lawrence, Kansas: Allen Press Inc., 459463.
Matsuura, K. 1979. Phylogeny of the Superfamily Balistoidea (Pisces: Tetraodontiformes). Memoirs of the Faculty of Fisheries, Hokkaido University, 26(1/2):49169.
Matsuura, K. and Tyler, J.C. 1994. Triggerfishes & their allies. In: Paxton, J.R. and Eschmeyer, W.N., edits. Encyclopedia of fishes. San Diego, California: Academic Press, 227231.
Miya, M., Takeshima, H., Endo, H., Ishiguro, N.B., Inoue, J.G., Mukai, T., Satoh, T.P., Yamaguchi, M., Kawaguchi, A., Mabuchi, K., Shirai, S.M., and Nishida, M. 2003. Major patterns of higher teleostean phylogenies: a new perspective based on 100 complete mitochondrial DNA sequences. Molecular Phylogenetics and Evolution, 26:121138.
Mok, H.-K. and Shen, S.-C. 1983. Osteology and phylogeny of Squamipinnes. Taiwan Museum Special Publication Series, Zoology, 1:187.
Nolf, D. and Tyler, J.C. 2006. Otolith evidence concerning interrelationships of caproid, zeiform and tetraodontiform fishes. Bulletin de lInstitut Royal des Sciences Naturelles de Belgique, Biologie, 76:147-189.
Rosen, D.E. 1984. Zeiforms as primitive plectognath fishes. American Museum Novitates, 2782:138.
Santini, F. and Tyler, J.C. 2002a. Phylogeny of the ocean sunfishes (Molidae, Tetraodontiformes), a highly derived group of teleost fishes. Italian Journal of Zoology, 69:3743.
Santini, F. and Tyler, J.C. 2002b. Phylogeny and biogeography of the extant species of triplespine fishes (Triacanthidae, Tetraodontiformes). Zoologica Scripta, 31:321330.
Santini, F. and Tyler, J.C. 2003. A phylogeny of the families of fossil and extant tetraodontiform fishes (Acanthomorpha, Tetraodontiformes), Upper Cretaceous to Recent. Zoological Journal of the Linnean Society, 139:565617.
Tyler, J.C. 1962. The pelvis and pelvic fin of plectognath fishes; a study in reduction. Proceedings of the Academy of Natural Sciences of Philadelphia, 114:207250.
Tyler, J.C. 1963. The apparent reduction in the number of precaudal vertebrae in trunkfishes (Ostraciontoidea, Plectognathi). Proceedings of the Academy of Natural Sciences of Philadelphia, 115:153190.
Tyler, J.C. 1968. A monograph on plectognath fishes of the superfamily Triacanthoidea. Monographs of the Academy of Natural Sciences of Philadelphia, 16:1364.
Tyler, J.C. 1980. Osteology, phylogeny, and higher classification of the fishes of the order Plectognathi (Tetraodontiformes). NOAA Technical Report NMFS Circular, 434:1422.
Tyler, J.C., Mirzaie, M., and Nazemi, A. 2006. New genus and species of basal tetraodontoid puffer fish from the Oligocene of Iran, related to the Zignoichthyidae (Tetraodontiformes). Bollettino del Museo Civico di Storia Naturale di Verona, 30:49-58.
Tyler, J.C., OToole, B., and Winterbottom, R. 2003. Phylogeny of the genera and families of zeiform fishes, with comments on their relationships with tetraodontiforms and caproids. Smithsonian Contributions to Zoology, 618:1110.
Tyler, J.C. and Santini, F. 2002. Review and reconstructions of the tetraodontiform fishes from the Eocene of Monte Bolca, Italy, with comments on related Tertiary taxa. Studi e Ricerche sui Giacimenti Terziari di Bolca, Museo Civico di Storia Naturale di Verona, 9:47119.
Tyler, J.C. and Sorbini, L. 1996. New superfamily and three new families of tetraodontiform fishes from the Upper Cretaceous: the earliest and most morphologically primitive plectognaths. Smithsonian Contributions to Paleobiology, 82:159.
Winterbottom, R. 1974. The familial phylogeny of the Tetraodontiformes (Acanthopterygii: Pisces) as evidenced by their comparative myology. Smithsonian Contributions to Zoology, 155:1201.
Winterbottom, R. and Tyler, J.C. 1983. Phylogenetic relationships of aracanin genera of boxfishes (Ostraciidae: Tetraodontiformes). Copeia, 1983(4):902917.
Title Illustrations

| Scientific Name | Aracana ornata |
|---|---|
| Location | captive at Shinagawa Aquarium, Tokyo, Japan |
| Specimen Condition | Live Specimen |
| Sex | Male |
| Source | ornate cowfish (ハコフグ) #1574 |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Maynard Hogg |
| Scientific Name | Melichthys vidua |
|---|---|
| Location | Molokini, Maui, Hawaii, USA |
| Specimen Condition | Live Specimen |
| Source | Triggerfish |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Pete Chen |
| Scientific Name | Diodon holocanthus |
|---|---|
| Location | captive at Umi-tamago Aquarium, Ōita, Japan |
| Specimen Condition | Live Specimen |
| Source | PICT2366.jpg |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Tetsushi Tsunemi |
About This Page

National Museum of Natural History, Smithsonian Institution

Johnson County Community College, Overland Park, Kansas, USA
Correspondence regarding this page should be directed to James C. Tyler at and Nancy Holcroft at
Page copyright © 2007 and
 Page: Tree of Life
Tetraodontiformes. triggerfishes, boxfishes, puffers (fugu), molas and allies.
Authored by
James C. Tyler and Nancy Holcroft.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Tetraodontiformes. triggerfishes, boxfishes, puffers (fugu), molas and allies.
Authored by
James C. Tyler and Nancy Holcroft.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 19 February 2007
- Content changed 19 February 2007
Citing this page:
Tyler, James C. and Nancy Holcroft. 2007. Tetraodontiformes. triggerfishes, boxfishes, puffers (fugu), molas and allies. Version 19 February 2007. http://tolweb.org/Tetraodontiformes/52153/2007.02.19 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site